In our previous blog, we talked about using AI for data management in clinical trials. We demonstrated its potential with the example of Jane — a clinical data manager tasked with finding whether an AE term (Febrile Neutropenia) matched the medication (Acetaminophen).
Our team at Saama understands the concerns surrounding AI. Some people may fear this technology removes the human element from the process. But when it comes to our solutions, we make sure there’s a “human in the loop” every single step of the way.
When a customer is using a Saama solution, every prediction is sent to the Clinical Data Manager for their “thumbs-up or thumbs-down” review.
Think of it like a Netflix show. You’re sitting on the couch at the end of the day and decide to relax with a show. You go through the list of recommendations (which, by the way, was created by Netflix’s algorithm).
How did they come up with this list? By keeping track of the shows you previously viewed — as well as their rating system. By rating the shows you watch, it improves their algorithm and recommends more shows according to your tastes.
The same principle applies to Saama’s AI solutions for clinical data management.
Once a prediction is received, a Clinical Data Manager can validate it (or not) using a simple two-step method:
Step 1: Validate ML prediction (answer “Yes” below) or reject ML prediction (answer “No” below).
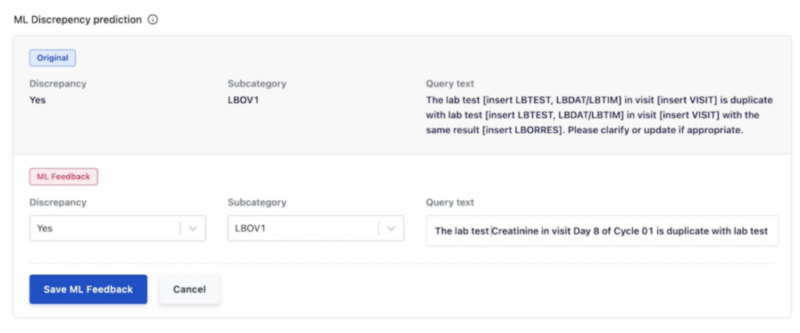
Step 2: Approve, reject or place on-hold the prediction and query text from Step 1.

Automated reviews (provided by our friendly AI technology) enhance both quality and efficiency for clinical data management. Here’s the process, along with the “how” which enables each step:
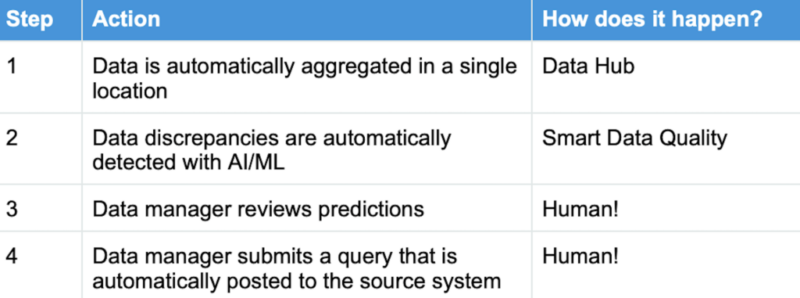
We have plenty of examples that show how Saama’s solutions “keep the human in the loop” — which we view as absolutely essential.
Next, we’ll begin our journey into understanding conversational AI, which you might know better as Chat GPT! Stay tuned to learn the power — and limitations — of this exciting new AI breakthrough, and how it can be used responsibly to accelerate research.